
▌ICH M7
"Assessment and Control of DNA Reactive (Mutagenic) Impurities in Drugs to Limit Potential Carcinogenic Risks" (ICH HARMONISED GUIDELINE--ASSESSMENT AND CONTROL OF DNA REACTIVE (MUTAGENIC) IMPURITIES IN PHARMACEUTICALS TO LIMIT POTENTIAL CARCINOGENIC RISK-M7(R1)) was released in February 2013 and was adopted by ICH regulatory members and recommended by ICH regulatory agencies in May 2017.
The guideline aims to provide a feasible framework for the identification, classification, qualification and control of genotoxic impurities to limit potential carcinogenic risks. It is intended to supplement ICH Q3A(R2), Q3B(R2) and M3(R2) to support non-clinical safety studies in human clinical trials and drug marketing licenses.
▌Genotoxic impurity
Genotoxic impurities refer to DNA reactive substances that may directly cause DNA damage at lower levels, lead to DNA mutations, and may cause cancer. Most of them are electrophiles, such as nitrosamines.
◆ Genotoxic Impurities: Genotoxic impurities, GTI.
◆ Potential Genotoxic Impurities: Potentially genotoxic impurities, PGI.
▌Genotoxic Impurities
According to ICH M7 guidelines, genotoxic impurities can be identified by two methods:
Search carcinogenicity and genotoxicity data (mutagenicity) through databases and literatures (CPDB,IRIS,NTP,ECHA, etc.)
were calculated using a (quantitative) structure activity relationship (QSAR). QSAR method was used to predict the results of bacterial mutation test (AMES) for toxicity evaluation. Two QSAR prediction methods are used: one is based on expert rule-based and the other is based on statistical-based. The QSAR model conforms to the OECD guidelines. If the results of the two models are consistent, the results are considered reliable, and if the results are inconsistent, supporting data needs to be provided for expert analysis.
▌Genotoxic Impurities
According to the hazard assessment results obtained from the literature database or QSAR calculation, impurities can be divided into 5 categories, and the detailed classification is shown in the table below.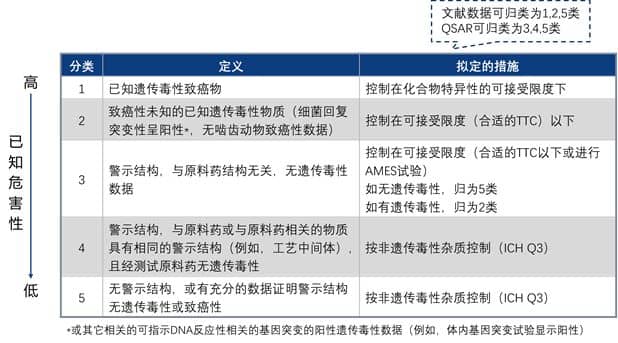
▌Genotoxic Impurities
◆ TTC: Calculate the acceptable intake of genotoxic impurities using the Threshold of Toxicological Concern, TTC) method
◆ Specificity limit: acceptable intake of compound specificity can be calculated based on rodent carcinogenic data, such as TD50 value; Benchmark dose can also be used instead of TD50 value as a quantitative indicator of carcinogenic titer, such as benchmark dose limit 10%(BMDL10).
◆ LTL: Shorter than the life cycle (Less-Than-Lifetime), the acceptable intake of 1.5 μg/d based on TTC is regarded as a protective daily life exposure.
◆ CoC: Cohort of Concern. Some structural groups are determined to have high carcinogenicity. Even if the intake is lower than the TTC level, there is still a potential significant carcinogenic risk in theory. Such highly effective genotoxic carcinogens are called "attention cohort".
▌Our service
◆ ICH M7 impurity QSAR prediction report
◆ Genotoxic Impurities
◆ Genotoxic Impurities
◆ Query similar objects
◆ Nitrosamines Impurities
◆ AMES test proxy
< Previous:Pesticide registration QSAR service
(+86)571-86060701
157875742
infor@jirehstandard.com

