
Recently, ECHA issued recommendations to registrants on how to reliably combine proxy data from different sources when assessing the skin sensitization of chemicals. The recommendations are based on a new OECD Guideline on Defined Approaches for Skin Sensitisation, developed by the OECD Expert Group on Defined Approaches for Skin Sensitisation (EG DASS). for Skin Sensitisation; EG DASS). The guidelines contain a clear methodology for assessing whether a substance is a skin sensitizer and classify the degree of sensitization.
This classification is important because REACH requires an assessment of the efficacy of skin sensitisation. If such determination methods (defined approaches, DAs) are used to reach conclusions on skin sensitization and potency, they will replace the currently used in vivo lymph node tests (LLNAs), thus reducing testing on animals.
Skin sensitizers (skin sensitiser) are a class of substances that produce a skin sensitisation reaction following repeated skin exposure. Skin sensitization reactions are generally due to the covalent binding of the sensitizer to proteins in the body. It is a process initiated by a molecule that produces a series of key events (KEs) that ultimately cause allergic dermatitis. The molecular initiation is the first key event (KE1), which begins with the covalent binding of an electrophilic substance in the skin protein to the nucleophilic center, i.e. protein binding. The second key event (KE2) occurs in keratinocytes and includes an inflammatory response and changes in gene expression associated with specific cellular signaling pathways, such as antioxidant/electrophoretic response element (ARE)-dependent pathways, known as keratinocyte activation. The third key event (KE3) is dendritic cell activation, which is usually assessed by the expression of specific cell surface markers, chemokines and cytokines. The fourth critical event (KE4) is the proliferation of T cells, which ultimately causes allergic contact dermatitis.
According to the guideline, the approach for sensitization determination (DA) is to use a defined set of information sources to generate a fixed data interpretation procedure (DIP) (e.g., mathematical models, rule-based methods) and apply it to the target substance to derive predictions without expert judgment. Information sources (data) for sensitization judgments are generally derived from in vitro chemical assays (in chemico), in vitro tests (in vitro), and computer simulation evaluations (in silico). Data from multiple sources of information can be used together in DAs to obtain the same or better predictive power as animal tests to predict human responses.The combination of methods in DAs can overcome some of the limitations of individual stand-alone methods to improve the confidence in the overall results obtained.
Before using DAs, the testing laboratory should consider all relevant information about the test chemical, such as the identifying characteristics of the test chemical (CAS, SMILES, etc.), chemical structure, physicochemical properties, toxicological data (if available), etc., to determine whether the particular DA is applicable to the test chemical.
The DAs contain a comprehensive dataset of 196 chemicals, including DA prediction data, personal information source data, highly accurate LLNA and human patch prediction test (HPPT) data, and physicochemical property data. Of these chemicals, 168 have LLNA classifications and 66 have HPPT classifications, which are approved by EG DASS and used to evaluate the performance of DAs. The dataset contains mainly cosmetic ingredients, but also other types of chemicals used across domains, such as preservatives, dyes or food ingredients. The chemical composition of the dataset is diverse, as indicated by the physicochemical properties covered by these chemicals: it contains small and large molecules (molecular weight range 30 to 512 g/mol), hydrophobic and hydrophilic substances (log P range -3.9 to 9.4), solids and liquids (melting point range -122 to 253ºC), volatile and non-volatile substances (boiling point range -19 to 445ºC), etc.
▌The DAs recommended in this guideline are currently of three types.
◆ "Three Takes Two" (2o3) strategy
for assessing skin sensitization hazards (divided into negative and positive). KE1: protein binding (direct peptide reactivity assay (DPRA)); KE2: keratinocyte activation (KeratinoSens™); KE3: dendritic cell activation (human cell line activation test (h-CLAT)).2o3 The data interpretation procedure (DIP) in DA is a transparent, rule-based approach that requires no expert judgment. The strategy predicts skin allergy risk by means of three internationally recognized non-animal analysis methods related to KE1-3 (i.e. DPRA, KeratinoSens™, h-CLAT) in an undefined sequence of consecutive tests. Two KEs were analyzed and if the results of these analyses were consistent, the chemicals were predicted to be negative or positive accordingly. If the results of the first two analyses are inconsistent, the analyses for the remaining KEs are run.
◆ Integrated Testing Strategy (ITSv1) based on UN GHS potency classification
Used to assess skin sensitization hazards and potency (divided into GHS 1A, 1B, and NC, i.e., strong sensitization, moderate to weak sensitization, and no classification). The strategy includes KE1 and KE3 in the KEs causing skin sensitization and includes computational simulation prediction of skin sensitization (in silico). Protein binding (KE1) was quantitatively assessed using DPRA and scored 0-3 by the strength of sensitization; h-CLAT quantitatively assessed dendritic cell activation (KE3) and scored 0-3 by the strength of sensitization; and (Q)SAR software Derek Nexus (ITSv1) provided computational simulation predictions of skin sensitization, scored 0-1 by the presence or absence of sensitization. CLAT can be evaluated when one of these methods is available, and the most reliable and accurate results are obtained when all three methods are available. The specific classification methods are as follows.
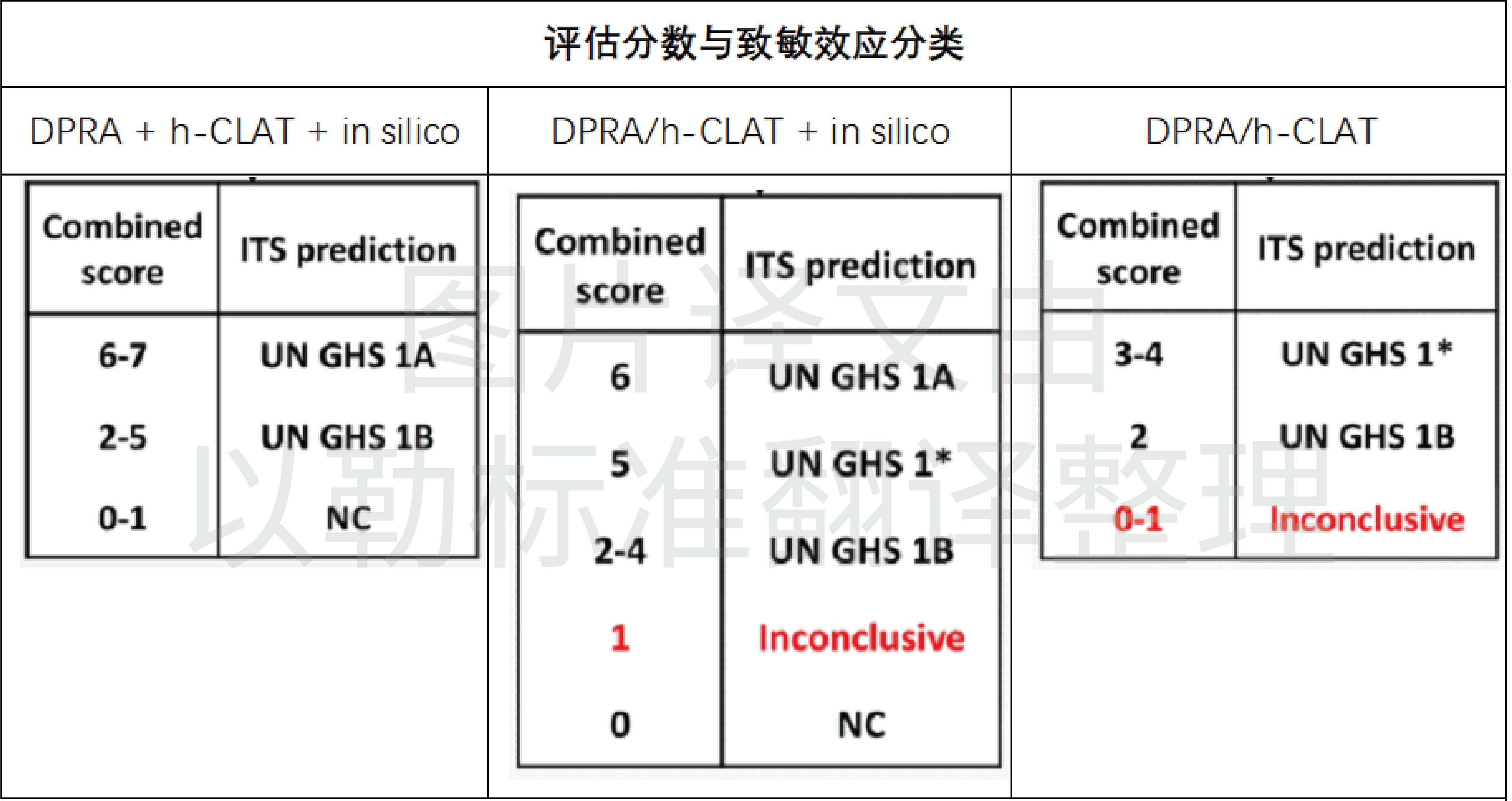
◆ Based on the ITSv1 modified strategy (ITSv2)
Used to assess skin sensitization hazards and efficacy (divided into GHS 1A, 1B and NC, i.e. strong sensitization, moderate to weak sensitization, no classification). This method changes the computational simulation prediction method in ITSv1 from Derek Nexus software to the OECD QSAR Toolbox. the assessment execution strategy is essentially the same as ITSv1.
▌ Selected prediction performance data for the three methods are as follows.
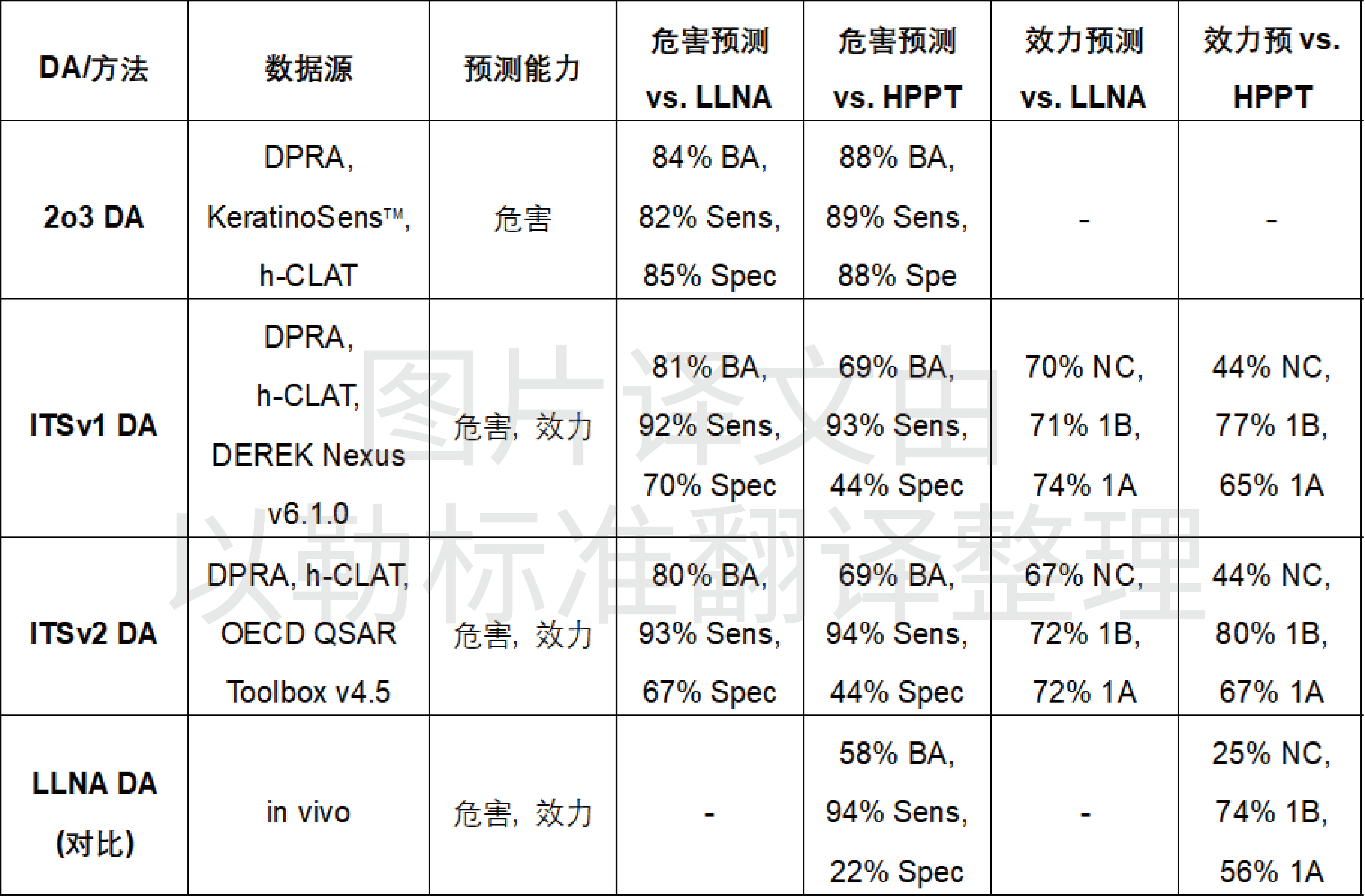
Note: Sensitivity (Sens) indicates the true positive rate, specificity (Spec) is the true negative rate, and balanced accuracy (BA) is the average of sensitivity and specificity. Due to the unbalanced nature of the reference data, different classification results of the same method differ.
All three DAs have high accuracy, and in the process of specific use, appropriate methods and strategies can be selected to achieve higher prediction accuracy by combining with the actual material situation. Some of the DAs are not applicable to metals, inorganic compounds, UVCB and mixtures. In addition, the sensitivity of DAs such as DPRA, KeratinoSens™, and h-CLAT is relatively low when LLNA data are used to run the prediction of substances in the Log P > 3.5 category, and the use of LLNA data is more prone to false-positive results than HPPT data, i.e., the current number of reference substances is not sufficient to draw conclusions with a high degree of certainty for substances with Log P > 3.5.
In addition, individual analytical results near the critical judgment range may yield inconclusive DA predictions (low confidence), and the guideline describes the critical judgment range for each DA. For example, the DPRA method has a critical range of 4.95% - 8.32% for results, which is initially determined as negative when the peptide chain depletion is less than 4.95% and positive when it is greater than 8.32%. In order to avoid incorrect judgments due to the critical range, both KeratinoSens™ and h-CLAT methods require multiple runs. In the DPRA method, multiple runs are also required to obtain exact results when peptide chain depletion is between 3-10% (or 9%-17% when using only cysteine). For example, if the result of the first run is 3%-4.95%, a second run is performed, and if the result of both runs is negative, the final result is negative, and if the two runs do not agree, a third run is performed and the result of the same two runs is taken as the final result (negative, positive or borderline).
For the final hazard/effect classification, DA predictions with high confidence are considered deterministic and DA predictions with low confidence are non-deterministic (Inconclusive). For example, negative h-CLAT results obtained for chemicals with Log P > 3.5 have low confidence and may lead to inconclusive 2o3 DA predictions. The guideline also specifically describes the final hazard/effect classification for the different DA confidence results.
The strategies ITSv1 and ITSv2 use the QSAR prediction tools Derek Nexus and OECD QSAR Toolbox, respectively, and the use of QSAR predictions is only necessary if at least one of the DPRA and h-CLAT methods is available; otherwise neither of these DAs can be used. The guidelines also explain the use and scope of application of both QSAR forecasting tools.
In general, this OECD guideline for skin sensitization determination combines the principles of multiple methods for skin sensitization determination of unknown substances, which ensures accuracy and is not too difficult to operate, and can solve the problem of sensitization determination without animal testing, which is in line with the humanitarian spirit and saves the testing period. ECHA has approved this method and recommended registrants to actively use this method to obtain data. Relevant REACH registrants can also respond positively and apply this method to the registration of actual products.
(+86)571-86060701
157875742
infor@jirehstandard.com

